

Reviving access to essential antibiotics
eCTD dossier and product supply for:
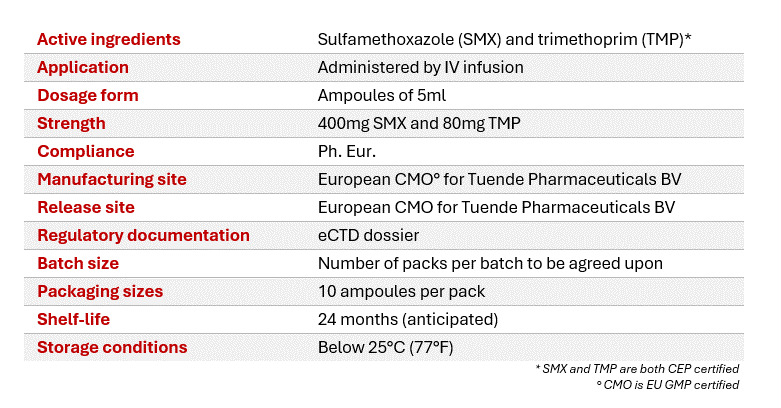
Co-trimoxazole
Solution for infusion, 5ml ampoules
Co-trimoxazole effectively treats a variety of bacterial infections, including urinary tract infections, respiratory infections, gastrointestinal infections, skin and soft tissue infections, and pneumonia.
Tuende is actively seeking commercial partners to register, market and distribute co-trimoxazole across Europe and other selected non-EU territories. Through Licensing & Supply Agreements, Tuende extends opportunities for eCTD dossier licensing and product supply.
The 2024-upgraded co-trimoxazole eCTD dossier developed by Tuende, based on the reference product Bactrim®, is prepared for submission to regulatory authorities for Marketing Authorization. Manufactured at a GMP-certified production facility based in Europe, the product ensures quality and compliance, featuring secured supply chain and eliminating the need for import testing.
For more information about commercial partnership opportunities, contact us at info@tuendepharma.com

Tuende Pharmaceuticals BV
Newtonplein 41
5283JH Boxtel
The Netherlands
Company registration number: KVK 85843474